Bursting Money Bins, the ice and water structure
📝 Original Info
- Title: Bursting Money Bins, the ice and water structure
- ArXiv ID: 1604.06563
- Date: 2016-04-26
- Authors: Franco Bagnoli
📝 Abstract
That water expands when freezing is a well-known fact, and it is at the basis of an experiment that is often involuntary performed with beer bottles in freezers. But why does the water behave this way? And, more difficult, how can one illustrate this phenomenon in simple terms?💡 Deep Analysis
📄 Full Content
Franco Bagnoli,
Dept. of Physics and Astronomy and Center for the Study of Complex Dynamics, University of
Florence, Italy
Via G. Sansone, 1 50019 Sesto Fiorentino (FI) Italy
franco.bagnoli@unifi.it
In the classic comics by Carl Barks, “The Big Bin on Killmotor Hill” [1], Uncle Scrooge, trying to defend his money bin from the Beagle Boys, follows a suggestion by Donald Duck, and fills the bin with water. Unfortunately, that night is going be the coldest one in the history of Ducksburg. The water freezes, bursting the ``ten-foot walls’’ of the money bin, and finally the gigantic cube of ice and dollars slips down the hill up to the Beagle Boys lot.
That water expands when freezing is a well-known fact, and it is at the basis of an experiment that is often involuntary performed with beer bottles in freezers. But why does the water behave this way? And, more difficult, how can one illustrate this phenomenon in simple terms?
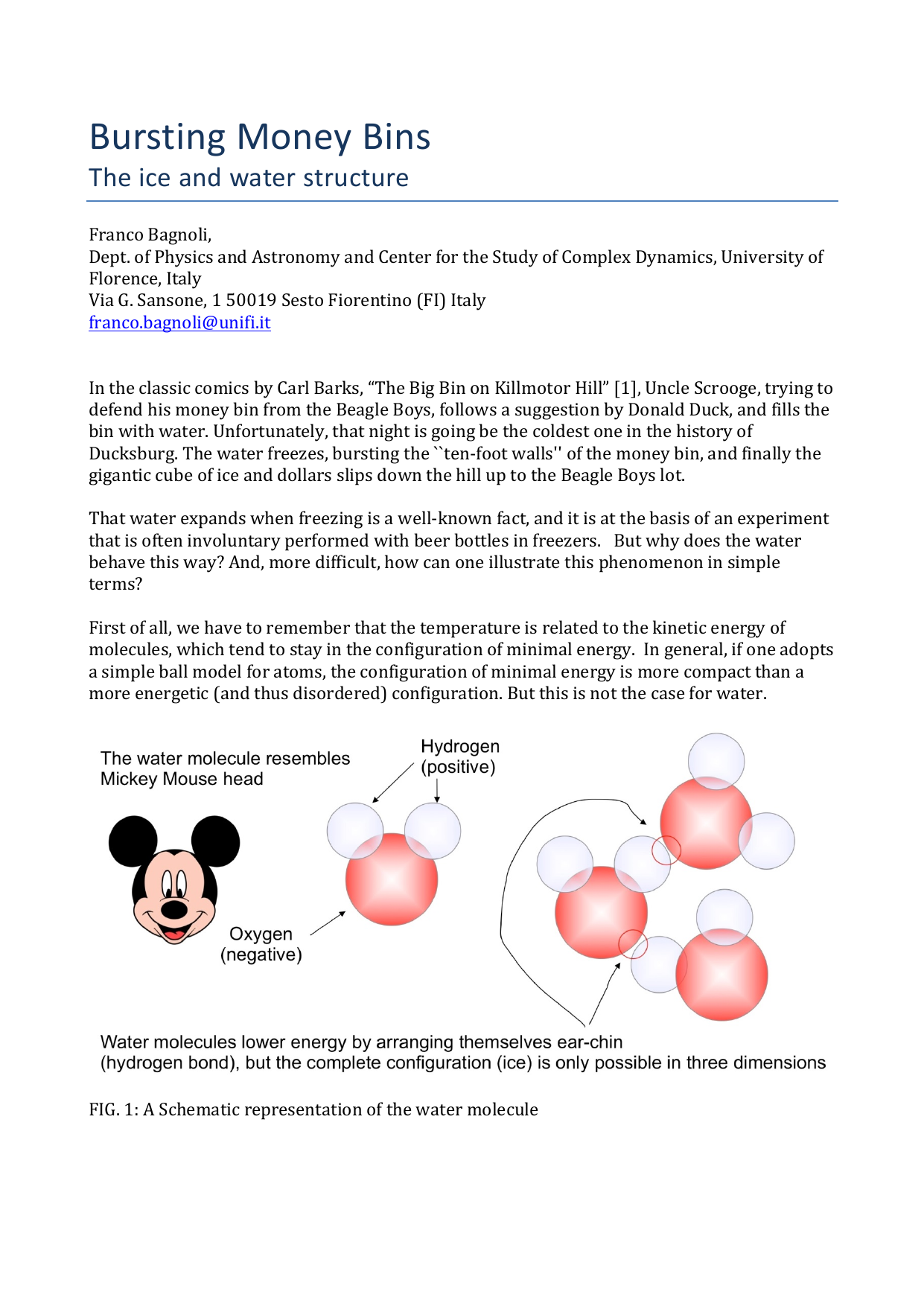
First of all, we have to remember that the temperature is related to the kinetic energy of molecules, which tend to stay in the configuration of minimal energy. In general, if one adopts a simple ball model for atoms, the configuration of minimal energy is more compact than a more energetic (and thus disordered) configuration. But this is not the case for water.
FIG. 1: A Schematic representation of the water molecule
Indeed, water molecules resemble the head of Mickey Mouse (see Fig. 1), the two hydrogen atoms being the ears. It is difficult to draw the crystal structure of water, since it is intrinsically three-dimensional, but one can make use of the ``Mercedes Benz’’ model [2].
FIG. 2: Water molecules in the Mercedes-Benz model
In this model, the water molecules are represented as the famous Mercedes-Benz symbol (see Fig. 2), and the minimum energy is obtained when two spikes join (an unoriented version of the hydrogen bond). It is not difficult to make some cardboard Mercedes-Benz molecules and let people play with them.
FIG. 3: Water and ice in the Mercedes-Benz model
By means of this model it is now easy to illustrate that the minimum energy configuration contains a lot of ``void’’ (see right-hand side of Fig. 3), and this explains the lower density of ice with respect to water. It may also serve to illustrate the phenomenon of regelation (melting under pressure and freezing again when the pressure is reduced).
However, in order to have a stable ice structure, we first have to reduce the kinetic energy of
water molecules, and then remove also the difference in energy from the Van der Waals to the
hydrogen bonds, i.e., we have to extract heat. In macroscopic terms, the first is called the heat
capacity of water (specific heat: C = 4.187 kJ/kg K), and the second the latent heat of melting (I
= 334 kJ/kg). If one wants to further lower the temperature of ice, one has also to extract the
energy corresponding to the specific heat of ice (2.108 kJ/kg K).
In Carl Barks’ comics, Uncle Scrooge often states that his money bin is “three cubic acres big”,
which is obviously nonsense, because the acre is a surface unit. If we assume that each face of
the cube is an acre (around 4,000 m2), we have a side of about 60 m, and a volume V of about
200,000 m3. I was unable to find the density of a random packing of coins (it seems that only
spheres and M&M’s have been studied [3]), so I arranged a small experiment with 174 ten
cents coins. The resulting packing density is about 0.55, much less than spheres (0.64), but
there are pronounced finite-size effects1. The total amount of water is thus W =
0.45×2×105×103 = 9×107 kg. Assuming that initially the money bin (and the water inside) is at
T = 10oC, the total amount of heat to be removed to obtain ice at 0oC is W × (C × T + I) =
3.4×1013 J if we disregard the heat capacity of coins – that of copper/brass is a mere 0.4 kJ/kg
K – and that of the walls, about 0.8 kJ/kg K. If the freezing occurs in, say, 5 hours (disregarding
the time required for the heat conduction), this means a cooling capacity of 113 GW! Indeed,
to freeze water is not an easy task, and it is a common practice to put cans full of water near
fruit trees in order to protect them from frost.
Finally, we close with a challenge: what is the temperature at the bottom of the sea?2
References
[1] http://coa.inducks.org/story.php?c=W+WDC+135-02
(1951). Available online on
http://it.paperpedia.wikia.com/wiki/File:Big_bin_on_killmotor_hill.pdf
[2] K.A.T. Silverstein, A.D.J. Haymet, K.A. Dill, A Simple Model of Water and the Hydrophobic
Effect, J. Am. Chem. Soc. 120, 3166-3175 (1998). doi:10.1021/ja973029k
[3] A. Donev, I. Cisse, D. Sachs, E.A. Varano, F.H. Stillinger, R. Connelly, S. Torquato, P.M.
Chaikin, Improving the Density of Jammed Disordered Packings Using Ellipsoids Science 303,
990-993 (2004), doi:10.1126/science.109
📸 Image Gallery
